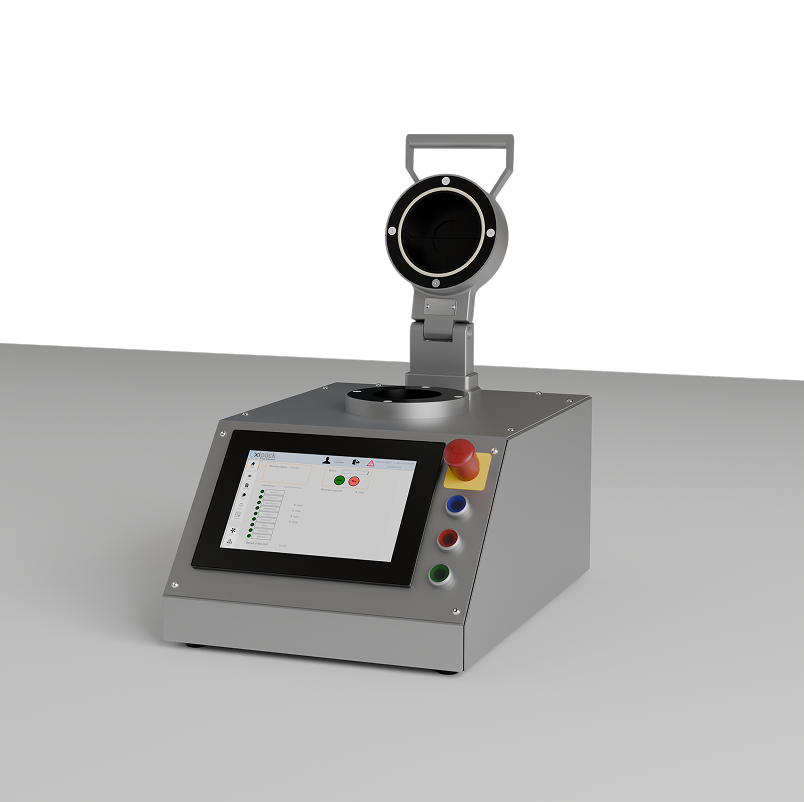









Container Closure Integrity Tester
The Oxipack Container Closure Integrity Tester (CCIT) is a high-precision leak testing solution tailored for the pharmaceutical and biological industries. Its advanced design allows it to adapt to a wide range of container types, such as vials, ampoules, and bottles. This flexibility leads to faster, more efficient testing.
The CCIT is distinguished by its high accuracy in leak inspection. It's special parts are designed to fit the packaging, creating a minimal measuring space and a deep vacuum in the test chamber. It delivers rapid performance, with a user-friendly touchscreen that provides a clear overview of settings and test results. Measuring leaks in pharmaceutical bottles via the vacuum decay method (ASTM F2338), the CCIT is an accurate, non-destructive, and straightforward solution for ensuring product integrity in sensitive industries.
Features
- Non-destructive vacuum decay method (ASTM F2338)
- 21 CFR Part 11 compliant software for secure electronic records and signatures.*
- Easy change parts in case of multiple shaped products.
- Integrated vacuum pump and control systems
Benefits
- Ideal for sensitive pharmaceutical products.
- Fast and accurate leak detection.
- Operator-independent results
- Suitable for diverse rigid products like pods, capsules, cans and bottles.



- Dimensions and Weight: 465 x 305 x 330 mm, 10 KG
- Materials: Powder coated steel, Anodized Aluminium, Polycarbonate, Onyx
- Power Supply: 100 - 240 VAC/kW
- Air Supply: > 5.5 - < 8 bar
- Air Consumption: 100 Nl/min
- Compliance and IP Rating: CE IP20, 21 CFR Part 11 (Software)
- Leak Detection Method: ASTM F2338
- Minimum Leakage: > 0,9 cm3/min
- Maximum Testing Capacity: 2P/M
- Connections: USB/Ethernet export, 24VDC logic (free programmable)
- Packaging Type and Size: Pods, capsules, cans, jars, bottles, vials, cups
*Compliance with 21 CFR Part 11:
- Electronic Records: Ensures secure and reliable storage of all test data, preventing unauthorized access or modifications.
- Audit Trail: All user actions and data entries are recorded with timestamped logs, supporting full traceability.
- Electronic Signatures: Supports digital signature functionality to verify and approve tests electronically.
- Access Control: Multi-level user authentication with role-based permissions to ensure that only authorized personnel can operate the SLT or modify critical settings.

Get in touch
Thank you for your submission!

Explore More Resources

The ROI of Switching to Non-Destructive Leak Detection
Switch to non-destructive leak detection and reduce waste, labor costs, and product loss. Learn how vacuum decay improves ROI and production efficiency.

